
Jim De Yoreo
University of Washington, USA
Title: Investigating materials formation with liquid-phase TEM
Biography
Biography: Jim De Yoreo
Abstract
Nucleation is the seminal process in the formation of ordered structures ranging from simple inorganic crystals to macromolecular matrices. Observations over the past 15 years have revealed a rich set of hierarchical pathways involving higher-order species ranging from multi-ion clusters to dense liquid droplets, as well as transient crystalline or amorphous phases. These non-classical pathways are diverse, in contrast to those of classical models that consider only addition of monomeric chemical species. Despite their complexity, a holistic framework for understanding particle-based pathways both during the nucleation and growth phases that extends classical concepts emerges when the coupled effects of complexity in free energy landscapes and the impact of dynamical factors governing particle formation and interaction are considered. Here, I describe that framework and use of a series of in situ TEM studies on inorganic and macromolecular systems to illustrate the evolution in nucleation and growth processes as these complexities and dynamical factors come into play. The introduction of either size-dependent phase stability associated with the high surface-to-volume ratios of nanoparticles, or high driving force coupled with the existence of metastable polymorphs leads to true two-step pathways characterized by the initial appearance of bulk precursor phases. Creation of micro-states, which represent local minima in free energy stabilized by configurational factors can also lead to hierarchical pathways, but the intermediates are transient states not present in the bulk phase diagram. In either of these cases, reduction in molecular mobility, either through reduced temperature or introduction of ion-binding macromolecules, can freeze non-equilibrium states into place for dynamical reasons. However, even when energy landscapes are smooth, high driving force creates dynamic factors that lead to hierarchical pathways through post-nucleation interaction and assembly of nanoparticles. Many of these processes can occur concurrently or sequentially in a single system.
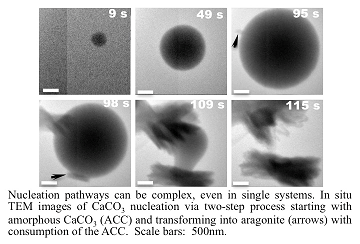
Recent Publications
- De Yoreo JJ, Sommerdijk N.A.J.M (2016) Investigating processes of materials formation via liquid phase and cryogenic TEM. Nature Mater. Reviews 1: 16035.
- De Yoreo JJ, Gilbert PUPA, Sommerdijk NAJM, Penn RL, Whitelam, S et al., (2015) Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments. Science 349: 498.
- Smeets PJM, Cho K-R, Kempen RGE, Sommerdijk NAJM, De Yoreo JJ, (2015) Calcium carbonate nucleation driven by ion binding in a biomimetic matrix revealed by in situ electron microscopy. Nature Mat. 14: 394-399.
- Nielsen MH, Aloni S, and De Yoreo JJ (2014) In situ TEM imaging of CaCO3 nucleation reveals coexistence of direct and indirect pathways. Science 345: 1158-1162.
5. Li D, Nielsen MH, Lee JR, Frandsen C, Banfield J, De Yoreo JJ, (2012) Direction-specific interactions control crystal growth by oriented attachment. Science 336: 1014-1018.

