
Kislon Voïtchovsky
Durham University, UK
Title: Dynamics of single ions and molecules at the surface of solids in solution
Biography
Biography: Kislon Voïtchovsky
Abstract
At the interface with solids, liquid tend to behave differently than in bulk. The interaction of the liquid molecules with the surface of the solid and their loss of configurational entropy often results in this interfacial liquid being more ordered and less mobile than its bulk counterpart, with dramatic consequences for the behavior of dissolved molecules and ions. The nanoscale organization and dynamics of interfacial liquids is key to countless processes from lubrication to protein function, heterogeneous catalysis, crystal growth and self-assembly. Experimentally little is known about the behavior of the interfacial liquid at the molecular level, partly for lack of technique able to gather in-situ local information, including over inhomogeneous interfaces. Atomic force microscopy (AFM) can in principle overcome this difficulty and provides sub-nanometer maps of the solvation landscape and the local solid-liquid affinity. Because the measurement is dynamical, more information can be derived about the nanoscale flow of the liquid parallel to the solid. Here, I present studies investigating the unusual dynamics of ions and small molecules at the interface with minerals in solution using a combination of high-resolution AFM and molecular dynamics simulations. I show that hydration water can drive the selfassembly of counter-ions, control adsorption of molecules such as stearate and nitrate, and dominate the surface restructuring of the solid. Tracking the dynamics of single atomic sites in solution shows remarkably slow changes ranging in the millisecond time-scale. Interfacial self-assembly of the liquid itself can also be achieved, for example, when using homogenous solutions comprising two pure liquids such as water and alcohol. The resulting solid-like nanostructures are remarkably stable and comprise both types of molecules. These structures can be exploited for controlled self-assembly and the development of functional interfaces.
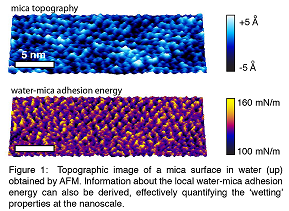
Recent Publications
1. Voïtchovsky K, Giofrè D, Segura JJ, Stellacci F, Ceriotti M (2016) Thermally-nucleated self-assembly of water and alcohol into stable structures at hydrophobic interfaces. Nature Commun. 7:13064
2. Hofmann S, Voïtchovsky K, Spijker P, Schmidt M, Stumpf T (2016) Visualising the molecular alteration of the calcite (104) – water interface by sodium nitrate. Sci. Rep. 6:21576
3. Ricci M, Segura JJ, Erickson BW, Fantner G, Stellacci F, Voïtchovsky K (2015) Growth and Dissolution of Calcite in the Presence of Adsorbed Stearic Acid. Langmuir 31:7563-7571
4. Ricci M, Spijker P, Voïtchovsky K (2014) Water-induced correlation between single ions imaged at the solid–liquid interface. Nature Commun. 5:4400
5. Ortiz-Young D, Chiu HC, Kim S, Voïtchovsky K, Riedo E (2013) The interplay between apparent viscosity and wettability in nanoconfined water. Nature Commun. 4:2482
6. Voïtchovsky K (2013) Anharmonicity, solvation forces, and resolution in atomic force microscopy at the solid-liquid interface. Physical Rev. E 88:022407

